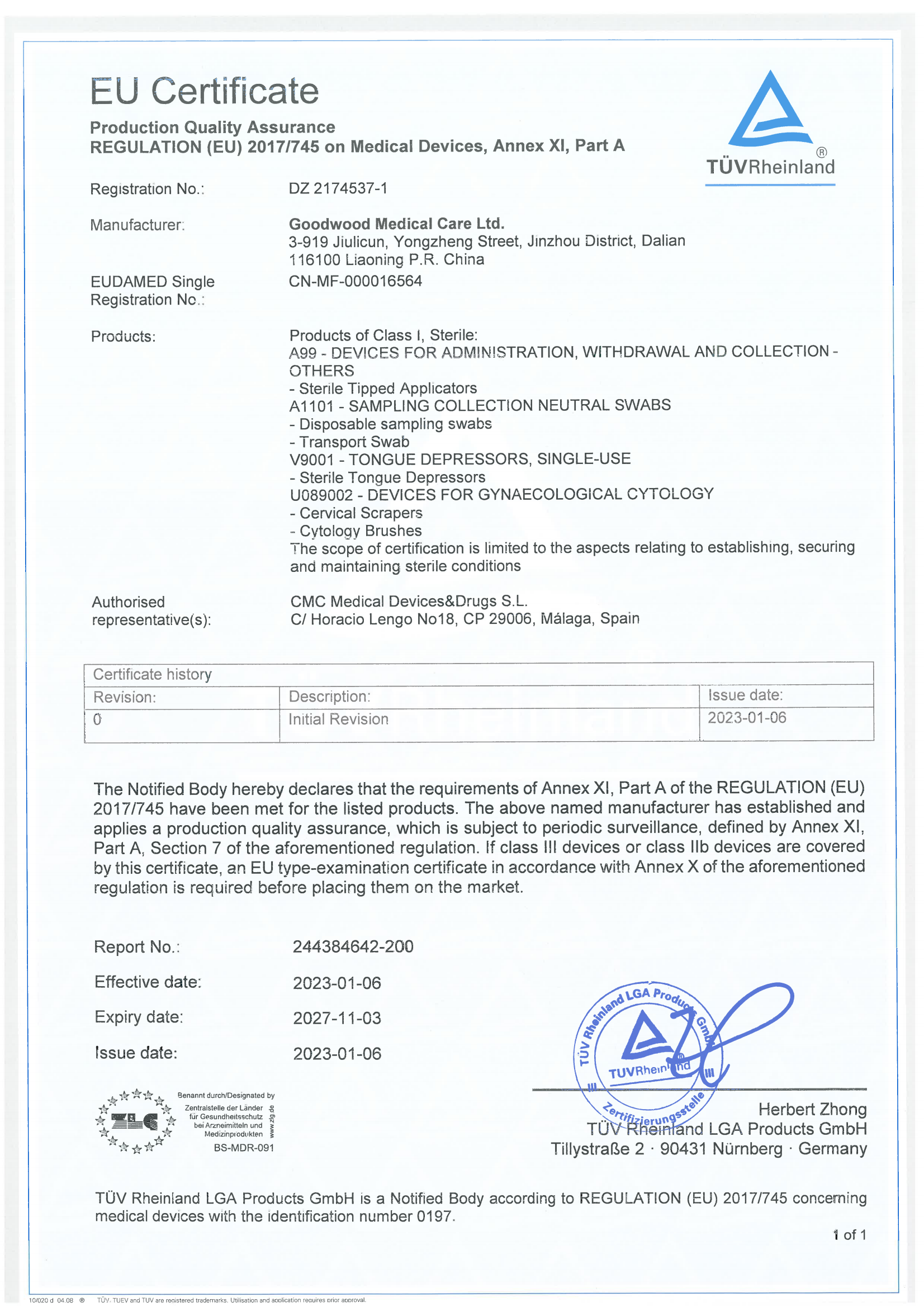
EU Certificate
Item specifics
- Period
- 2023/1/6 - 2027/11/3
- No.
- 244384642-200
- Certification bodies
- CMC Medical Devices&Drugs S.L.
Certificate description
The Notified Body hereby declares that the reguirements of Annex X!, Part A of the REGULATION (EU)2017/745 have been met for the listed products. The above named manufacturer has established andapplies a production quality assurance, which is subject to periodic surveillance, defined by Annex Xl.Part A, Section 7 of the aforementioned regulation. lf class ll devices or class llb devices are coveredby this certificate, an EU type-examination certificate in accordance with Annex X of the aforementionedregulation is required before placing them on the market.